One pill makes you larger and one pill makes you small. The ones that the Food and Drug Administration give us don't do anything at all.
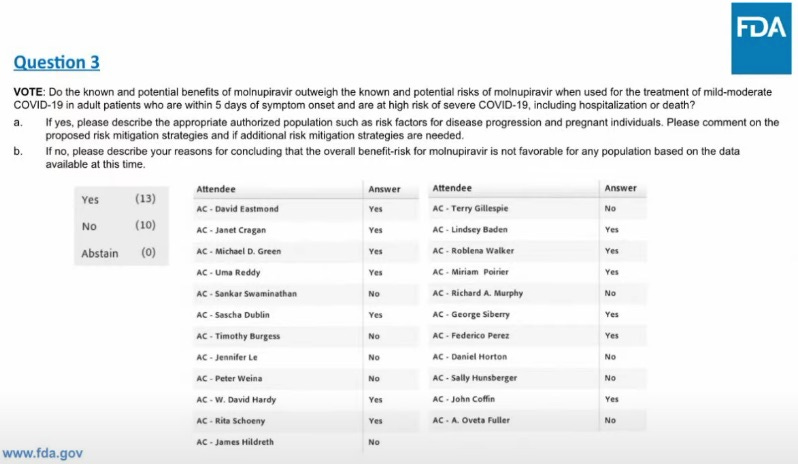
The United States Food and Drug Administration has officially approved Merck’s Molnupiravir for Emergency Use Authorization. Oh boy, this should be a controversial one. On November 30th, an FDA panel of advisors narrowly approved the recommendation for EUA in a 13-10 vote.
A split decision should give absolutely no one solace. Why would anyone take an EUA drug that even FDA stooges could not make a unanimous decision on? Does anyone really think a pill so contentious is being evaluated entirely on its merits? I would bet if you went down the list of “yes” votes, it would not be hard to find at least two people that have financial interests in the matter. Maybe they have received a grant from Merck — or more likely, they understand that tanking the value of Merck’s shares will guarantee they never get one in the future. The fact that the FDA ran with such a contentious vote and still approved the drug is insane.
Efficacy
The interim results from the trial were “ok”. Neither great, nor bad. In total, the risk of hospitalization or death:
Placebo cohort: 53/377 (14.1%)
Molnupiravir cohort: 28/385 (7.3%)
Relative risk reduction: 48.27%
Absolute risk reduction: 6.79%
The final results tell a completely different story.
Placebo cohort: 68/699 (9.7%)
Molnupiravir cohort: 48/709 (6.8%)
Relative risk reduction: 30.4%
Absolute risk reduction: 2.96%
In other words, since the interim results, the difference between the two groups was negative for Molnupiravir. Here is the difference:
Placebo cohort: 15/322 (4.66%)
Molnupiravir cohort: 20/324 (6.17%)
Relative risk reduction: -32.51%
Absolute risk reduction: -1.51%
Even for the final results, Merck estimates the confidence intervals for absolute risk at 0.1% to 5.9% with 95% confidence. In other words, barely statistically significant. This difference could easily be made up in the underlying characteristics of the two cohorts, which I will mention in regards to safety. More men in the placebo group alone, considering men often have worse outcomes with COVID-19, would be enough to make these results absolutely worthless.
Let’s talk about the above in the next section.
Safety
The potential risks from Molnupiravir are disturbing. These risks include bone marrow and leukemia risks to creating new, deadlier strains of the virus through mutagenesis. Interestingly, there are no obvious red flags from the trial results, possibly due to the fact that individuals were only follow for 29 days. But no obvious red flags may be a red flag in and of itself. You may call me an eternal skeptic, but I find it interesting that a placebo would apparently lead to higher rates of adverse events than an 800-mg dose of Molnupiravir.
“Adverse events were reported in 216 of 710 participants (30.4%) in the Molnupiravir group and 231 of 701 (33.0%) in the placebo group".
The placebo used in the trials is not described anywhere in the main findings or supplementary materials, so I will not go there. The real question this begs, in my opinion, is whether something went wrong in randomization. We are assured by Merck that the two groups were similar except for gender (more males were in the placebo group), but other than a few summary statistics, we are faced with a situation where Merck has asymmetric information. We cannot see, for example, the severity of the risks in each group.
It may be a good time to note that only “high-risk” individuals participated in the trials. One way to play around with randomization would be to throw obesity into the same category as cancer and consider the two an equal risk factor. Oh, look, that’s exactly what Merck did. Here are the risk factors from the interim analysis where Molnupiravir outperformed the placebo.
The first column is the Molnupiravir cohort versus the placebo cohort in the second column. The third and final column is the total.
Obesity, for once, is actually a benefit in these trials. In the period between the interim analysis, because they have the even out the risk factors, these numbers switch. There is no table presented, but I have reconstructed the above table for the period between the interim analysis and the final results.
And the worst part is the risk factors never fully even out. The Molnupiravir cohort is over-represented in obesity and under-represented in other categories once you add up these two periods. If Merck had done a study on a placebo versus a placebo, they probably could have arrived at the same results. This drug is garbage.





The second-half negative efficacy during the trial was impressive, but seems to be more a factor of the placebo group suddenly performing better while Molnupiravir severe outcome rate was steady. Doesn't seem to matter. The drug probably doesn't make a huge difference to infection outcomes, it's just a scapegoat for the cancers that the Covid-vaccinated were already going to get anyway.
The Organic Consumers Association and National Vaccine Information organisation today got excluded from Paypal. They can no longer get donation through Paypal. That desparate is the government to get the poison in all that they try to cut off everyone who does not comply.